Tuning the selectivity of catalytic nitriles hydrogenation by structure regulation in atomically dispersed Pd catalysts
Research Scientist LIU Hongyang, his Master’s student LIU Zhibo, and Post-doctor HUANG Fei from Shenyang National Laboratory of Materials Science, cooperated with Prof. MA Ding from Peking University, Prof. SUN Hongbin from Northeastern University, Prof. WANG Ning from Hong Kong University of Science and Technology, Research Scientist WEN Xiaodong from Institute Coal Chemistry, CAS, and their teams, to construct an atomically dispersed and fully exposed Pd clusters and single Pd atoms catalyst to tune the selectivity of catalytic nitriles hydrogenation.
The results were recently published online by Nature Communications (https://www.nature.com/articles/s41467-021-26542-y).
Amines, both primary and secondary amines, are important raw materials in the synthesis of bioactive molecules in pharmaceuticals synthesis. There are many methods developed and utilized in both industry and academia to obtain amines, such as amination of aryl halides or alcohols, reductive amination of aldehydes or ketones, hydroamination of olefins or alkynes, hydrogenation of nitriles, alkylative amination, and basepromoted mono-N-alkylation. Among them, hydrogenation of nitriles has attracted extensive attention. However, owing to the high thermodynamic stability of nitriles, hydrogenation of nitriles to target chemicals is difficult. Therefore, it is quite desirable to develop highly effective catalysts, especially those that can precisely control the product selectivity towards nitrile hydrogenation.
Research Scientist Liu Hongyang and his team have dedicated years into the study of precise design of metal catalyst at atomic scale and its application. Building on previous research, the team fabricated two kinds of atomically dispersed catalysts, single Pd atoms (Pd1/ND@G) and fully exposed Pd clusters (Pdn/ND@G), both of which are immobilized on the defect-rich nanodiamond-graphene hybrid support as the aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) images, the X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) analysis revealed (Fig.1). Subsequently, the catalytic performances and selectivities of Pd1/ND@G and Pdn/ND@G catalysts in transfer hydrogenation of benzonitrile was evaluated (Fig.2). DFT calculation (Fig.3) reveals that the intermediate BI is easier to further hydrogenate to BA on the Pdn/NDG catalyst. While on the Pd1/NDG catalyst, BI is more inclined to undergo condensation reaction and continuing hydrogenation to obtain DBA. The selectivity regulation strategy established over these catalysts with atomic precision in structure will pave the way for the rational design and construction of the highly selective catalyst with fully metal utilization efficiency. Currently, Research Scientist Liu Hongyang and his team are cooperating closely with enterprises to futher exploit and promoting this technology for its promising value in the chemical and pharmaceutical industries.
The present work is supported by the National Key R&D Program of China, the National Natural Science Foundation of China, Liaoning Revitalization Talents Program, Shenyang National Laboratory for Materials Science and Shanghai Synchrotron Radiation Facility.
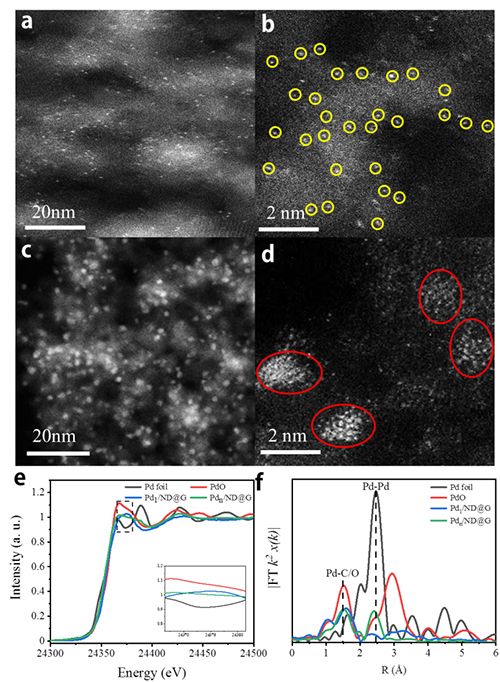
Fig. 1 Structural characterization of catalysts. (a, b) HAADF-STEM images of Pd1/ND@G; (c, d) HAADF-STEM images of Pdn/ND@G. (e) Pd K-edge XANES profiles and (f) EXAFS spectra for Pd1/ND@G and Pdn/ND@G.
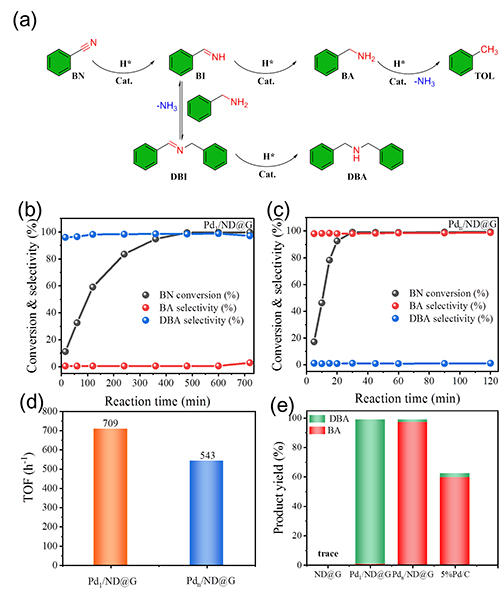
Fig. 2 Catalytic performances of Pd1/ND@G and Pdn/ND@G catalysts in transfer hydrogenation of benzonitrile.
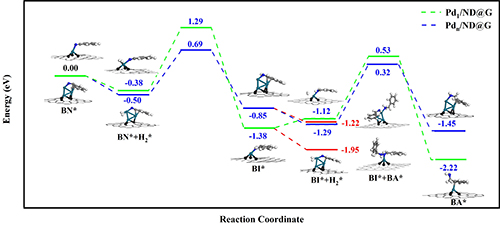
Fig. 3 Step-by-step hydrogenation mechanism of benzonitrile to benzylamine on Pd1/ND@G and Pdn/ND@G.
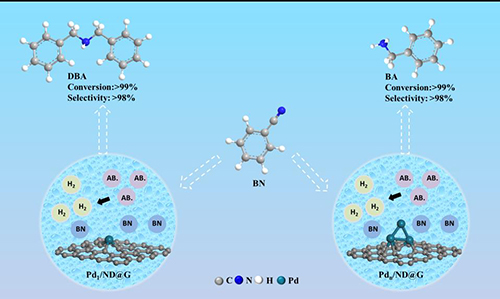
Fig. 4 The catalytic transfer hydrogenation of nitriles by atomically dispersed single Pd atoms and fully exposed Pdn clusters as active centers.